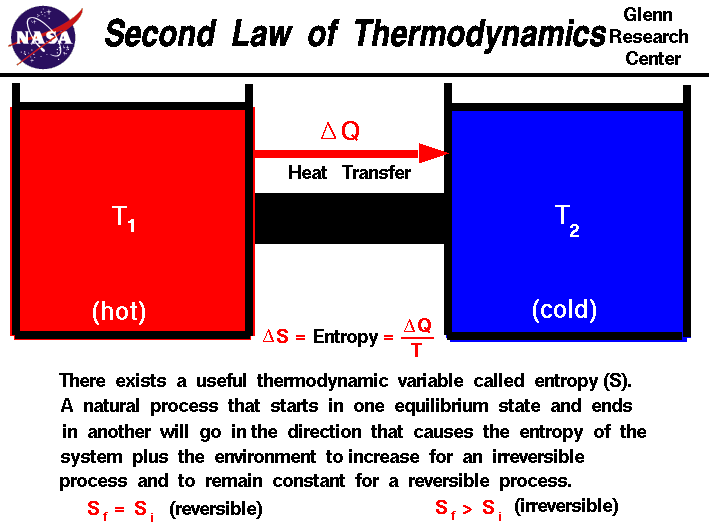
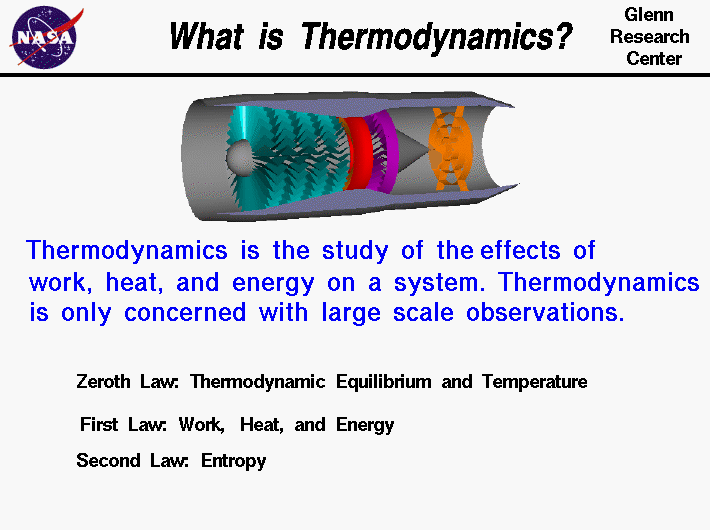
The Second Law of Thermodynamics relates the heat associated with a process to the entropy change for that process. The second law of the thermodynamics we are going to talk about the quality of the energy.
Second Law Of Thermodynamic Authorstream
However no information about the direction of the process can be obtained by the application of the first law.

What is the second law of thermodynamics. Where the first law states about the Quantity of energy. The second law of thermodynamics is a physical law that is not symmetric to reversal of the time direction. The second law also states that the changes in the.
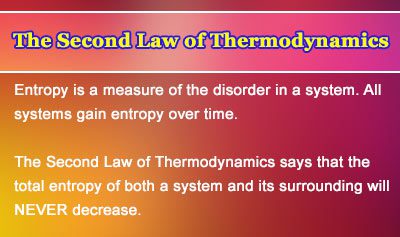
The second law of thermodynamics states that in a natural thermodynamic process the sum of the entropies of the interacting thermodynamic systems never decreases. Heat transfer occurs spontaneously from higher- to lower-temperature bodies but never spontaneously in the reverse direction. In chemistry the second law of thermodynamics is mainly focused on entropy.
In all the spontaneous processes the entropy of the universe increases. Observer T hermodynamics is the study of heat and energy. The second law of thermodynamics is a general principle that goes beyond the limitations imposed by the first law of thermodynamics.
Therefore as a redox reaction proceeds there is a heat change related to the extent of the reaction dqdks TdSdks. The first law is used to relate and to evaluate the various energies involved in a process. This does not conflict with symmetries observed in the fundamental laws of physics particularly CPT symmetry since the second law applies statistically on time-asymmetric boundary conditions.
The Second Law of Thermodynamics says that processes that involve the transfer or conversion of heat energy are irreversible. The second law of thermodynamics states that the entropy of any isolated system always increases. The first and second law are the most frequently used laws in thermodynamics.
Entropy statement of Second law of thermodynamics. Kelvin Plancks statement of second law of thermodynamics says that there must be at least two thermal reservoirs to operate the engine. The Second Law of Thermodynamics is one of the Thermodynamic laws from the three laws of thermodynamics.
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. What is the Second Law of Thermodynamics. The second law on the other hand asserts that some thermodynamic processes are forbidden.
The first law says that energy can be neither created nor destroyed. Another form of the statement is that heat does not spontaneously pass from a colder body to a warmer body. The entropy statement of the second law of thermodynamics is given below.
Hayati Kayhan Shutterstock The laws of thermodynamics. Thermo meaning heat and dynamic meaning power. Thus the Laws of Thermodynamics are the Laws of Heat Power As far as we can tell these Laws are absolute.
The Second Law of Thermodynamics is one of three Laws of Thermodynamics. The Second Law of Thermodynamics first expression. The second law of thermodynamics says that when energy changes from one form to another form or matter moves freely entropy disorder in a closed system increases.
A machine that violated the first law would be called a perpetual motion machine of the first kind because it would manufacture its own energy out of nothing and thereby run forever. The Second Law of Thermodynamics states that the state of entropy of the entire universe as an isolated system will always increase over time. At its heart are laws that describe how energy moves around within a system whether an atom.
The first law is simply another version of the law of conservation of energy. The second law of thermodynamics states that any spontaneously occurring process will always lead to an escalation in the entropy S of the universe. Which means the energy neither be created nor it can be destroyed.
The term thermodynamics comes from two root words. Due to the force of gravity density and pressure do not even out vertically. Differences in temperature pressure and density tend to even out horizontally after a while.
The law states that it is impossible for any process to have as its sole result heat transfer from a cooler to a hotter object. In simple words the law explains that an isolated systems entropy will never decrease over time. The second law of thermodynamics The first law of thermodynamics asserts that energy must be conserved in any process involving the exchange of heat and work between a system and its surroundings.
This is the Law of Conservation Energy. The second law of thermodynamics Photograph.
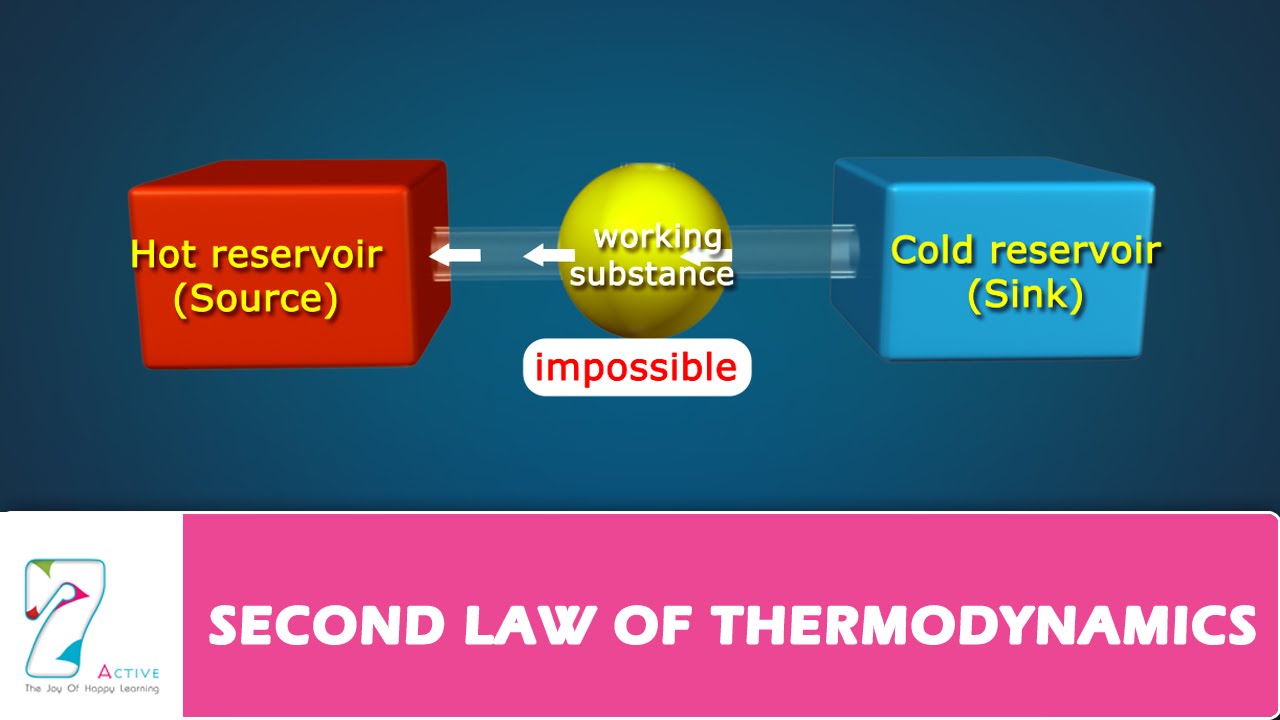 Second Law Of Thermodynamics Youtube
Second Law Of Thermodynamics Youtube
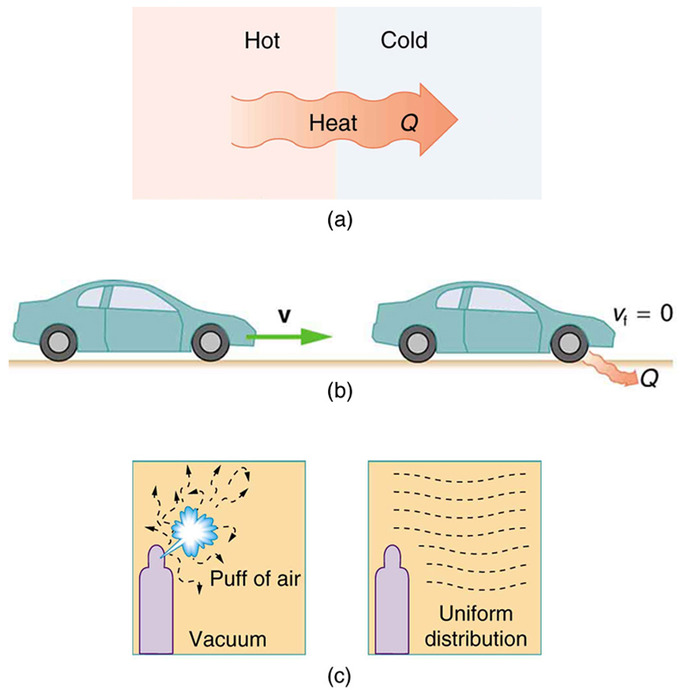 The Second Law Of Thermodynamics Boundless Physics
The Second Law Of Thermodynamics Boundless Physics
 The Laws Of Thermodynamics Thermodynamics Chemistry Education Engineering Science
The Laws Of Thermodynamics Thermodynamics Chemistry Education Engineering Science
What Is The Second Law Of The Thermodynamics Statement Quora
 Second Law Of Thermodynamics Video Khan Academy
Second Law Of Thermodynamics Video Khan Academy
 Second Law Of Thermodynamics Heat Energy Entropy Spontaneous Processes Youtube
Second Law Of Thermodynamics Heat Energy Entropy Spontaneous Processes Youtube
 The Social Implications Of The Second Law Of Thermodynamics Gene Veith
The Social Implications Of The Second Law Of Thermodynamics Gene Veith
 Chapter 5 The Second Law Of Thermodynamics Learning Outcomes Demonstrate Understanding Of Key Concepts Related To The Second Law Of Thermodynamics Ppt Download
Chapter 5 The Second Law Of Thermodynamics Learning Outcomes Demonstrate Understanding Of Key Concepts Related To The Second Law Of Thermodynamics Ppt Download
 What Is The Original Formula For The Second Law Of Thermodynamics Quora
What Is The Original Formula For The Second Law Of Thermodynamics Quora