This term is typically used only when the liquid surface is in contact with gas eg air. Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible.
What is surface tension.

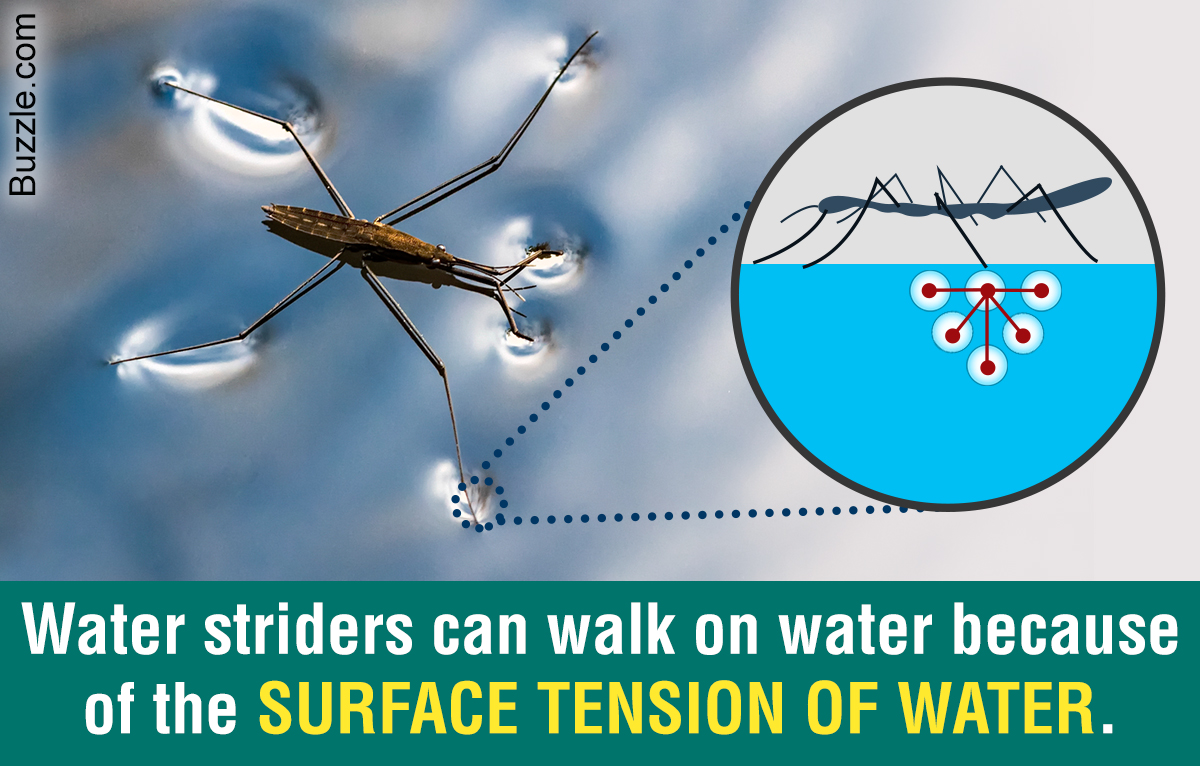
Surface tension of water. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension of water can cause things to float which are denser than water allowing organisms to literally walk on water Figure PageIndex2. The definition of surface tension is the work that must be applied in order to enlarge the surface of a phase.
Surface Tension of Water. Surface tension can be defined as. This is a table of surface tension values for some interfaces at the indicated temperatures.
An example of such an organism is the water strider which can run across the surface of water due to the intermolecular forces of the molecules and the force of the strider which is distributed to its legs. Surface Tension of Water. They press very gently on the surface of the water so as not to break through it.
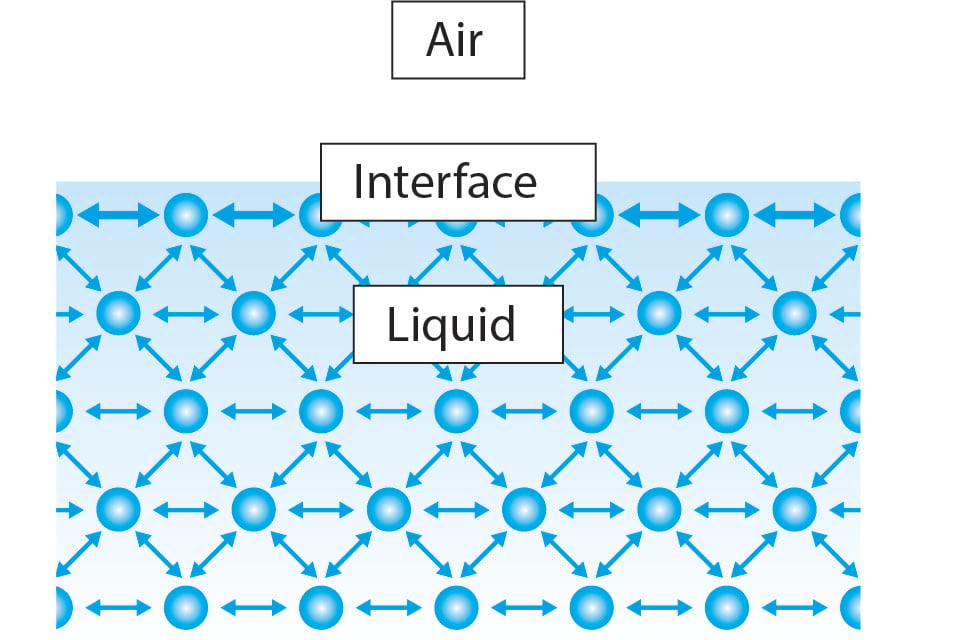
If the surface is between two liquids eg water and oil it is called interface tension. Since these intermolecular forces vary depending on the nature of the liquid. Surface tension is the energy or work required to increase the surface area of a liquid due to intermolecular forces.
The surface tension of water is about 72 mNm at room temperature which is one of the highest surface tension for liquid. There is only one liquid having higher surface tension and thats mercury which is a liquid metal with the surface tension of almost 500 mNm. The shape that has the smallest possible area for a given volume is a sphere.
The surface tension of water is an important parameter for many biological or industrial processes and roughly a factor of 3 higher than that of nonpolar liquids such as oils which is usually attributed to hydrogen bonding and dipolar interactions. When a pin is delicately placed on the surface of still water it creates a small depression on the waters surface. These pond skaters have long hairy legs which allow them to spread their weight over a wide area.
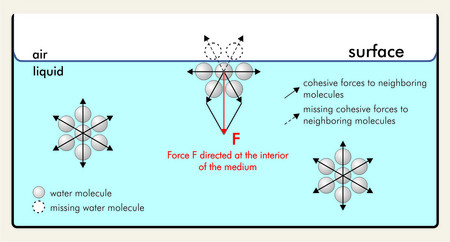
Surface tensiondecreases with increasing temperature of the liquid and its critical temperature is equal to zero. At liquidair interfaces surface tension results from the greater attraction of liquid molecules to each other due to cohesion than to the molecules in the. A platinum-iridium ring is drawn out of the liquid while at the same time the maximum force caused by the tension of the liquid lamella during the movement of the ring is measured.
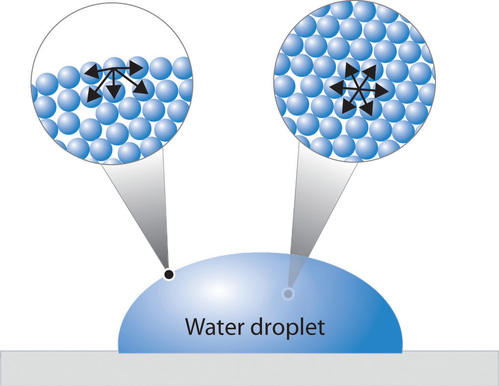
In comparison organic liquids such as benzene and alcohols have lower surface tensions whereas mercury has a higher surface tension. The surface tension arises from the polar nature of the water molecule. Surface tension is the energy required to stretch a unit change of surface area - and the surface tension will form a drop of liquid to a sphere since the sphere offers the smallest area for a definite volume.
That means a drop of water will want to have the smallest possible surface area. This term is typically used only when the liquid surface is in contact with gas such as the air. If the pin is of unit length then through out its length the waters surface experiences a force T.
If the surface is between two liquids such as water and oil it is called interface tension. Table water surface tension at the air boundary. Surface tension is caused by the inward attraction of molecules at a boundary.
Surface Tension of Water in contact with Air - s-10-3 lbft 32. It is the high surface tension of water which allows insects to walk over it. The surface tension of water is 72 dynescm at 25C.
Water Surface Tension Surface tension is a phenomenon in which the surface of a liquid where the liquid is in contact with the gas acts as a thin elastic sheet. Note that the SI units millinewtons per meter mNm 1 are equivalent to the cgs units dynes per centimetre dyncm 1. The pin floats on the surface of water because of waters surface tension.
Water has a high surface tension because the water molecules on the surface are pulled together by strong hydrogen bonds. Surface tension is a force which causes a layer of liquid to behave like an elastic sheet or skin. The Du Nouey ring method is used to determine the surface tension of a liquid.
For many liquids formula applies-ddt d Mp23 212 M- molecular weight p- the density t- temperature. The surface tension of water decreases significantly with temperature as shown in the graph. Water striders to float and slide on a water surface without becoming even partly submerged.
Water has a surface tension of 007275 joule per square metre at 20 C 68 F. Surface tension is a phenomenon in which the surface of a liquid where the liquid is in contact with a gas acts as a thin elastic sheet. Surface tension allows insects eg.
An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Surface tension also allows for the formation of droplets that we see in nature. The experimental data shown in these pages are freely available and have been published already in the DDB Explorer EditionThe data represent a small sub list of all available data in the Dortmund Data BankFor more data or any further information please search the DDB or contact DDBST.
 The Meaning Of Surface Tension And Its Practical Applications Science Struck
The Meaning Of Surface Tension And Its Practical Applications Science Struck
/139802493-56a12f615f9b58b7d0bcde91.jpg) Surface Tension Definition In Chemistry
Surface Tension Definition In Chemistry
 Surface Tension Of Water Why Is It So High
Surface Tension Of Water Why Is It So High
/Surface-Tension-58c6c2365f9b58af5c534f71.jpg) What Is Surface Tension Definition And Experiments
What Is Surface Tension Definition And Experiments
Why Do Insects Float On Water According To The Concept Of Surface Tension Quora
 Surface Tension Chemistry Libretexts
Surface Tension Chemistry Libretexts
 Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube
Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube
 Values Of The Surface Tension Of Water At Different Temperatures 1 Download Table
Values Of The Surface Tension Of Water At Different Temperatures 1 Download Table
 Process Parameter Surface Tension Basics About Sita Process Solutions Information Service Sita Process Solutions
Process Parameter Surface Tension Basics About Sita Process Solutions Information Service Sita Process Solutions
 Surface Tension What Is Surface Tension Kibron
Surface Tension What Is Surface Tension Kibron


