Amorphous solids are rigid structures but they lack a well-defined shape. Gives clean sharp cleavage.
The key difference between amorphous and crystalline solid is that the crystalline solids have an ordered long-range arrangement of atoms or molecules within the structure whereas the amorphous solids lack ordered long-range arrangement.
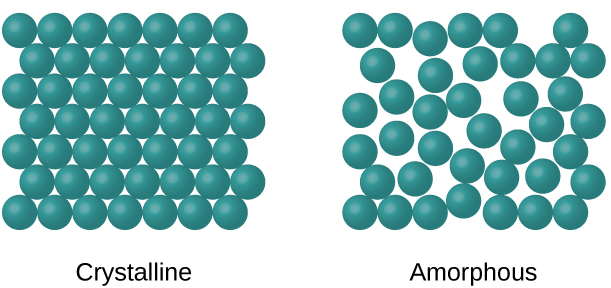
How is a crystalline solid different from an amorphous solid. Amorphous solid is isotropic which means that their physical properties are identical in all the directions. The most common example of an amorphous solid is Glass. The constituent particles are arranged in irregular three dimensional patterns.
So they are non-crystalline. They have definite heat of fusion. The constituent particles atoms ions or molecules are arranged in regular and definite three dimensional patterns.
Usually the amorphous solids exhibit irregular cut. Unlike crystalline solids they do not have a definite geometrical shape. They also give different edges when cut with a knife.
In addition to this main difference there are many more differences between these two types of solids. Amorphous solids do not have a regular external structure and they do not have sharp melting points. However in non-crystalline solids particles have a little freedom to move since they are not arranged rigidly as in other solids.
Crystalline solids are anisotropic in nature while amorphous solids possess isotropism. Amorphous solids do not possess any particular heat of fusion. Crystalline solids are very rigid and their molecules cannot be deformed by mild distorting force.
In condensed matter physics and materials science an amorphousfrom the Greeka without morphe shape form or non-crystalline solidis a solid that lacks the long-range order that is characteristic of a crystal. For example sodium chloride diamond sugar etc. The atoms in solids pack closely together than in liquids and gases.
Amorphous solid do not exhibit rigidity. We can classify solids into two as crystalline and amorphous depending on the atomic level arrangement. Crystalline solid is anisotropic which means that their physical properties are not identical in all directions.
Yes amorphous solids can be converted into crystalline solids depending on some factors and conditions. They do not have a geometric shape. This means the energy required to melt crystalline solids is fixed whereas it may vary in the case of amorphous solid.
Difference Between Crystalline and Amorphous Amorphous and crystalline are two states that describe typical solids in chemistry. The main difference between amorphous and crystalline solids is that amorphous solids do not have an ordered structure whereas crystalline solids have a highly ordered structure. This is why they do not have edges like crystals do.
Amorphous solids have atoms arrangement in an indefinite manner whereas crystalline solids have atoms arrangement in a definite manner. Difference Between Crystalline and Amorphous There are three states of matter namely solids liquids and gases. Using X-ray diffraction experiments the structure of solids can be categorized into crystalline or amorphous non-crystalline.
The shape of crystalline and amorphous solids Melting point- When Crystalline solid is heated after reaching the melting temperature it gets melted at the temperature means it has a sharp melting point whereas in case of amorphous solid it gets soften and melts over a range of temperature and does not have a sharp melting point. Characteristics of Amorphous Solids. Whereas an amorphous solid melts over a range of temperature.
Crystalline solids melt at a fixed temperature. In some older books the term has been used synonymously with glass. These small parts of amorphous solid behaving like crystalline solid are called crystallites.
First the microstructure of a solid material has potential talent and ability to form ordered structures to some extend. The particles are arranged with a definite or indefinite geometry. Amorphous solids give poor pattern when exposed to x-rays while crystalline solids give a good and definite pattern.
Crystalline solids exhibit a cleaner and distinct edge when they are cut with a knife while the amorphous solid show irregular patterns when cut with a knife. The arrangement of constituent particles atoms molecules or ions in such a solid has only short-range order. Properties of amorphous solids are different in many ways from those of crystalline solids.
The intermolecular force forces in amorphous solids are weaker than those in crystalline solids. This article explains 1. The general answer to this very general question is.
Deformation could be done by bending or compressing them. Solids are among the three basic states of matter that include liquids and gases. Amorphous solids are basically the exact opposite of crystalline solids.
Cutting with a knife. Non- crystalline solids are amorphous solids. Solids have two states namely amorphous and crystalline form.
While an amorphous solid may display some finite order in terms of the arrangement of its atoms ions and molecules it will clearly lack the long-range ordered structure that a crystalline solid exhibits.
 11 7 Amorphous Solids Ms Smith
11 7 Amorphous Solids Ms Smith
Difference Between Crystalline And Amorphous Difference Between
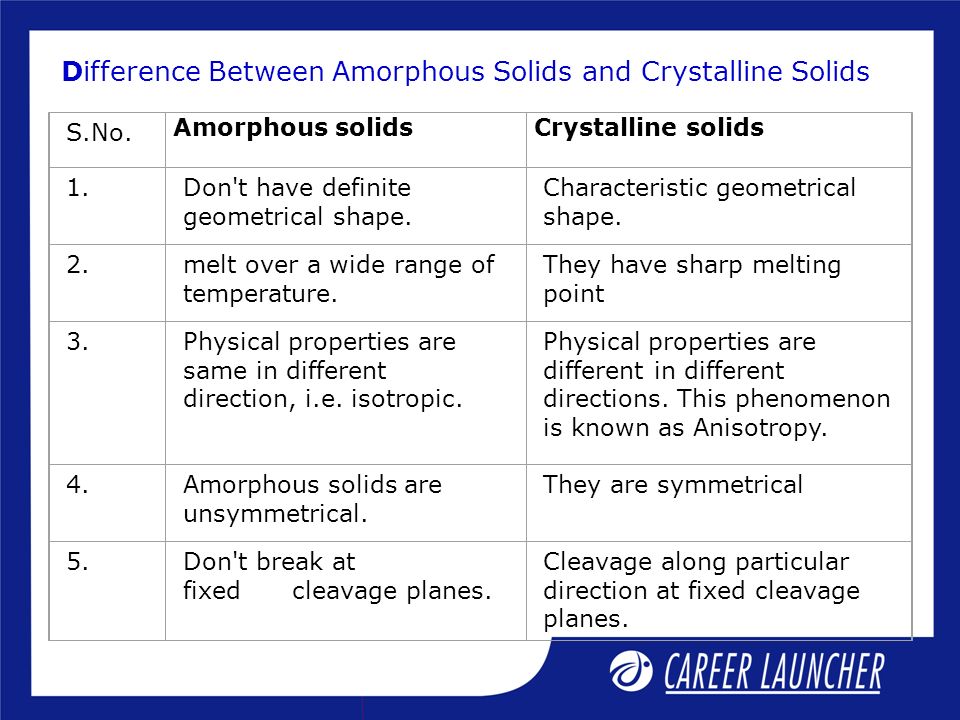 Crystalline And Amorphous Solids Ppt
Crystalline And Amorphous Solids Ppt
 Pin By Sofia On Study Crystalline Solid Nomenclature Chemistry Chemistry Class
Pin By Sofia On Study Crystalline Solid Nomenclature Chemistry Chemistry Class
 Crystalline Vs Amorphous Solids What S The Difference Science Struck
Crystalline Vs Amorphous Solids What S The Difference Science Struck
 Crystalline And Amorphous Solids Explanation Differences Examples Etc
Crystalline And Amorphous Solids Explanation Differences Examples Etc
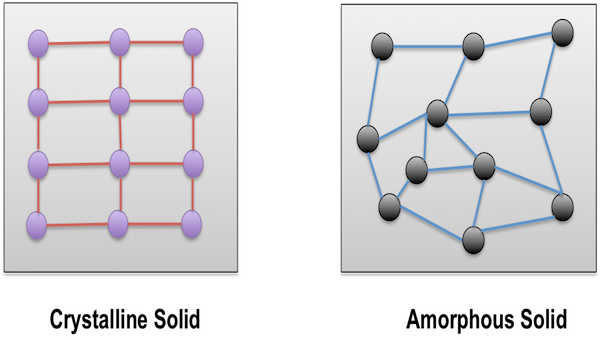 Amorphous And Crystalline Solids Study Material For Iit Jee Askiitians
Amorphous And Crystalline Solids Study Material For Iit Jee Askiitians
 Crystalline And Amorphous Solids Explanation Differences Examples Etc
Crystalline And Amorphous Solids Explanation Differences Examples Etc
 Difference Between Amorphous And Crystalline Solid Compare The Difference Between Similar Terms
Difference Between Amorphous And Crystalline Solid Compare The Difference Between Similar Terms
 12 4 The Fundamental Types Of Crystalline Solids Chemistry Libretexts
12 4 The Fundamental Types Of Crystalline Solids Chemistry Libretexts
Powerschool Learning 8th Grade Science Sec 1 Solids
.jpg) Difference Between Crystalline And Amorphous Solids Curlyarrows Chemistry Tutorials
Difference Between Crystalline And Amorphous Solids Curlyarrows Chemistry Tutorials
What Is The Difference Between A Crystalline And Non Crystalline Solid Which Factor Promotes Crystalline Solid Structure Quora
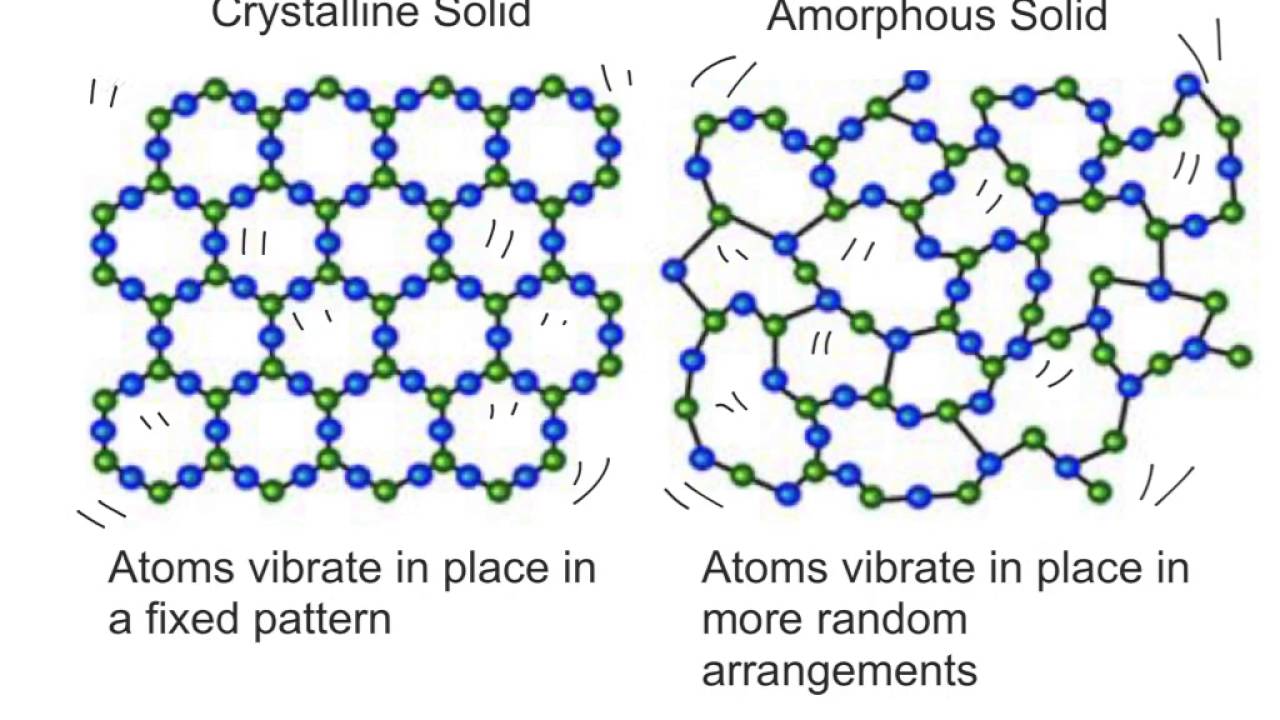 Amorphous And Crystalline Solids Youtube
Amorphous And Crystalline Solids Youtube