PALMAZ BLUE Stent Cordis A Johnson and Johnson Co. The litigation was settled when Boston Scientific agreed to pay 716 million to Johnson Johnson in September 2009 and an additional 173 billion in February 2010.
 New Johnson Johnson Johnson And Johnson Cordis Precise Pro Rx Nitinol Stent System X Pc0930rxc Disposables General For Sale Dotmed Listing 2716779
New Johnson Johnson Johnson And Johnson Cordis Precise Pro Rx Nitinol Stent System X Pc0930rxc Disposables General For Sale Dotmed Listing 2716779
Johnson Johnson announced on Wednesday that it would stop manufacturing drug-coated heart stents by the end of the year abandoning an intensely competitive 4 billion market.

Johnson and johnson stents. Cordis Corporation part of the Johnson Johnson family of companies is a worldwide leader in the development and manufacture of interventional vascular technology. With clinical follow-up out to four years CYPHER -- the only drug-eluting stent using the drug sirolimus has the longest-term data available of any drug-eluting stent. 81 Johnson Johnson Stent.
Johnson Johnson Stent. For more than 40 years Cordis Corporation a Johnson Johnson company has pioneered less-invasive treatments for vascular disease. A Johnson Johnson Enterprise Inc post frame building is designed to withstand 85 mph winds 3 feet of standing snow seismic 3 earthquake standards and have a maintenance-free exterior.
These included sanitary protection products. The Perfect Market-Dynamics Storm. Johnson Johnson JJ developed the first working stent a small medical implement that could be used for patients with artery blockages in lieu of open heart surgery.
The company focused on bandages sterile sutures and wound care. When stents were first introduced during the 1990s they were bare-metal devices inserted into the arteries. Johnson and Johnson Interventional NSN Parts.
Johnson Johnson has announced that it will be dropping one of its medical devices from production by the end of 2011 the company will cease manufacturing its drug-coated heart stents. The Perfect Market-Dynamics Storm. Through the companys innovation research and development Cordis works with interventional cardiologists interventional radiologists and vascular surgeons worldwide to treat millions of patients who suffer from vascular disease.
It also sold maternity kits with first-aid products to make childbirth safer. The tube-shaped coronary device that was inserted into arteries revolutionized the field of cardiology providing doctors with a new lifesaving tool. Johnson Johnson is looking to funnel the newly freed funds into developing other medical products.
Since 2003 Johnson Johnson and Boston Scientific have both claimed that the other had infringed on their patents covering heart stent medical devices. Johnson Johnsons Cordis bails on stents June 15 2011 By MassDevice staff One of the biggest players in the coronary stent market is getting out of the business. It was developed by Cordis Corporation which is a Johnson and Johnson Company.
Johnson Johnson JJ developed the first working stent a small medical implement that could be used for patients with artery blockages in lieu of open heart surgery. Johnson Johnson JJ developed the first working stent a small medical implement that could be used for patients with artery blockages in lieu of open heart surgery. A Johnson Johnson operating company released the worlds first coronary stent the PALMAZ-SCHATZ Balloon-Expandable Stent.
Johnson Johnson announced yesterday that it would stop making its line of drug-coated coronary stents and its exit from the 4 billion business would include halting the development of Nevo the newest version of its collection of tiny heart devices. Johnson Johnson Enterprise Inc can take on projects of any size and we are always happy to create a custom quote just for you. CRT30 PS1535 PS1530 PALMAZ-SCHATZ PALMAZ PS1540.
The company explains its decision was due its decreasing market share in the heart stent category. A tiny metal scaffold that is inserted into an artery during a balloon angioplasty procedure the stent. Cordis Corporation a Johnson Johnson company specializes in the development and manufacture of interventional vascular technology.
Johnson Johnson announced the trial results before stock trading began on Monday saying it would drop plans to seek federal approval to sell Conors stent the CoStar in the United States. About Cordis Corporation. The Johnson and Johnson Cypher stent is a sirolimus-drug-eluting stent which was introduced in 2003.
Through the companys innovation research and development Cordis partners with physicians worldwide to treat millions of patients who suffer from vascular disease. The company also sold womens health products. Robert James and Edward Johnson founded Johnson Johnson in 1886 in New Brunswick New Jersey.
A tiny metal scaffold that is inserted into an artery during a balloon angioplasty procedure.
 Medical Devices Products Johnson Johnson
Medical Devices Products Johnson Johnson
 Heart Stents Still Overused Experts Say The New York Times
Heart Stents Still Overused Experts Say The New York Times
 Implantation Of Palmaz Stent Johnson Johnson Cordis Haan Germany Download Scientific Diagram
Implantation Of Palmaz Stent Johnson Johnson Cordis Haan Germany Download Scientific Diagram
 Johnson Johnson Exiting 4 Billion Drug Coated Stent Business Nj Com
Johnson Johnson Exiting 4 Billion Drug Coated Stent Business Nj Com
 Peripheral Stent Palmaz Genesis On The Cylinder Opta Pro 0 035 Cordis Johnson And Johnson The Usa Ukrspecsnab Ooo All Biz
Peripheral Stent Palmaz Genesis On The Cylinder Opta Pro 0 035 Cordis Johnson And Johnson The Usa Ukrspecsnab Ooo All Biz
 Palmaz Schatz Balloon Expandable Coronary Stent History Johnson Johnson Our Story
Palmaz Schatz Balloon Expandable Coronary Stent History Johnson Johnson Our Story
 Johnson Johnson Moving Out Of Stent Business Restructuring Cordis Unit Nj Com
Johnson Johnson Moving Out Of Stent Business Restructuring Cordis Unit Nj Com
Stent Wars Tales Of Defensive Strategy In The Medical Device Market Leaders Blog
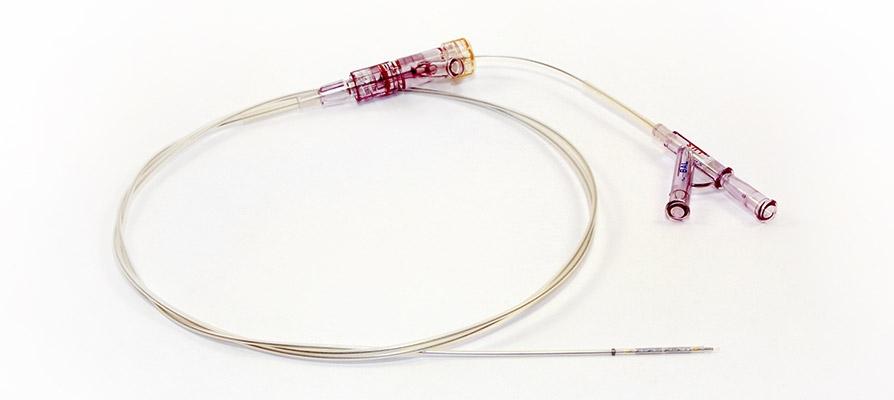 Palmaz Shatz Balloon Expandable Stent With Delivery System By Johnson Johnson
Palmaz Shatz Balloon Expandable Stent With Delivery System By Johnson Johnson
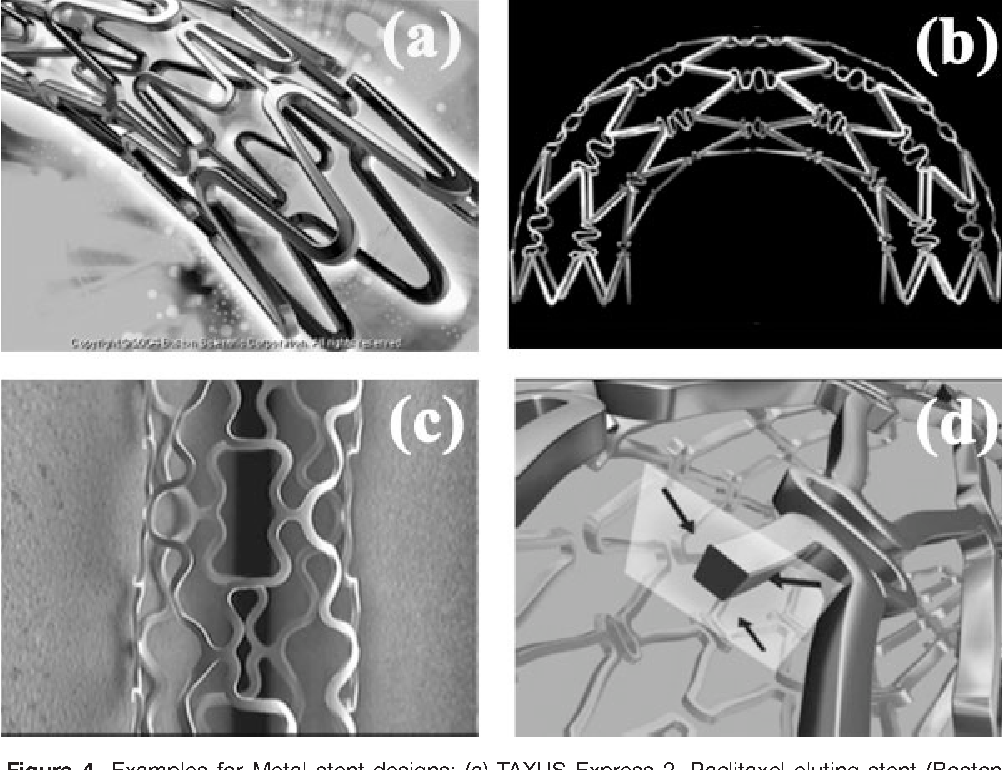 Figure 4 From Approaches For Prevention Of Restenosis Semantic Scholar
Figure 4 From Approaches For Prevention Of Restenosis Semantic Scholar
Cordis S M A R T Stent Still Looking Good At 3 Years Massdevice


